Ironmaking methods mainly include blast furnace method, direct reduction method, smelting reduction method, etc. the principle is that the reduced pig iron is obtained by physicochemical reaction of ore in a specific atmosphere (reducing substances Co, H2, C; appropriate temperature, etc.). In addition to a small part of pig iron used for casting, the vast majority is used as steel-making raw materials.
Blast furnace ironmaking is the main method of modern ironmaking and an important link in iron and steel production. Due to good technical and economic indicators, simple process, large production capacity, high labor productivity and low energy consumption, iron produced by blast furnace process accounts for more than 95% of the world’s total iron production.
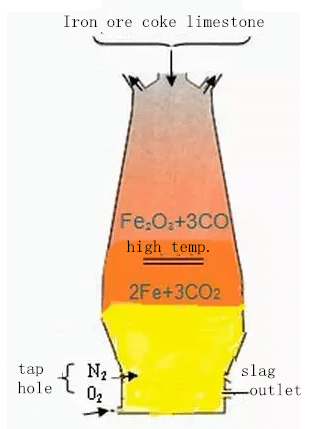
Schematic diagram of blast furnace ironmaking
Blast furnace is similar to a cylindrical furnace, its outside is covered with steel plate, and its inner wall is lined with firebrick. The whole furnace is built on a deep concrete foundation.
During the production of blast furnace, iron ore, coke and slag making flux (limestone) are loaded from the top of the furnace, and preheated air is blown into the tuyere located at the lower part of the furnace along the circumference of the furnace. At high temperature, carbon monoxide and hydrogen generated by the combustion of carbon in coke and oxygen blown into the air remove oxygen from iron ore in the process of rising in the furnace, so as to obtain iron. The molten iron is discharged from the taphole.
Non reducing impurities in iron ore combine with limestone and other fluxes to form slag, which is discharged from slag port. The gas produced is exported from the top of the furnace and used as the fuel of hot blast furnace, heating furnace, coke oven and boiler after dust removal.
Raw materials: iron ore, solvent, fuel
Iron ore
It is difficult to meet the requirements of blast furnace smelting in terms of chemical composition, physical state and other aspects of naturally mined ore. It must be prepared and treated by crushing, screening, beneficiation, briquetting and mixing to supply blast furnace with high grade, uniform composition and particle size.
There are four kinds of iron ore commonly used in metallurgical industry.
| Mineral Types | Main components | Theoretical content of Iron | 自然含鐵量 |
| Hematite | Fe2O3 | 70% | 50%~60% |
| magnetite | Fe3O4 | 72.4% | 40%~70% |
| limonite | 2Fe2O3·3H2O | 59.8% | 37%~55% |
| Siderite | FeCo3 | 48.2% | 低 |
solvent
Gangue in ore and ash in fuel contain some compounds with high melting point (for example, the melting point of SiO2 is 1625 ℃ and that of Al2O3 is 2050 ℃). They can not be melted into liquid at the smelting temperature of blast furnace, so they can not be well separated from molten iron. At the same time, the operation of furnace is difficult.
The purpose of adding flux is to form low melting point slag with these high melting point compounds, so as to completely liquefy at the smelting temperature of blast furnace and maintain considerable fluidity, so as to achieve the purpose of good separation from metal and ensure the quality of pig iron.
According to the properties of flux, it can be divided into basic flux and acid flux. Which flux to use depends on the properties of gangue in ore and ash in fuel. Since most gangues in natural ores are acidic and the ash content of coke is acidic, alkaline fluxes, such as limestone, are usually used. Acid fluxes are rarely used.
Fuel
The heat needed by blast furnace smelting mainly depends on the combustion of fuel. At the same time, the fuel also plays the role of reducing agent in the combustion process, so the fuel is one of the main raw materials for blast furnace smelting. The commonly used fuel is mainly coke, anthracite and semi coke.
Physical and chemical process: reduction reaction at high temperature + slagging reaction
The purpose of blast furnace smelting is to reduce iron from iron ore and remove impurities. In the whole smelting process, the most important is the reduction of iron and slagging reaction.
In addition, it is accompanied by a series of other complex physical and chemical reactions, such as evaporation of water and volatile matter, decomposition of carbonate, carbonization and melting of iron, reduction of other elements, etc., which can only be realized at a certain temperature. Therefore, the smelting process also needs fuel combustion as a necessary condition.
Combustion of fuel
C+O2→CO2
Decomposition of burden
Evaporation of water and decomposition of crystal water; elimination of volatiles; decomposition of carbonate.
Reduction reaction in blast furnace
Reduction of iron
In blast furnace, iron is not directly reduced from high valence oxide, but through a process of reduction from high valence oxide to low valence oxide, and then from low valence oxide to iron: Fe2O3 → Fe3O4 → FeO → Fe
The reduction of iron mainly depends on carbon monoxide gas and solid carbon as reducing agent. The reduction of carbon monoxide is usually called indirect reduction, and the reduction of solid carbon is called direct reduction.
The total reaction of indirect reduction is 3fe2o3 + 9co → 6fe + 9co2
The total reaction of direct reduction is 3fe2o3 + C → 2fe3o4 + Co
Carbonization of iron
The iron reduced from the ore is solid spongy, and its carbon content is very low, usually less than 1%. Because co decomposes at a lower temperature, and the decomposed C has a strong activity, when it contacts with iron, it is easy to form iron carbon alloy.
Therefore, the solid sponge iron begins to carburize at a lower temperature (400 ℃~ 600 ℃). The chemical reaction is as follows: 2CO + 3Fe → Fe3C + CO2 or 3Fe (liquid) + C (solid) → Fe3C
Slagging process
Slagging is a process in which gangue in ore and ash in fuel are combined with flux and removed from blast furnace. There are two kinds of slag formation in blast furnace
When smelting with ordinary acid ore, the flux is loaded into the blast furnace in the form of limestone, and the Cao in the flux can not be in close contact with the acid oxides in the ore. therefore, the slag initially formed is mainly fe2sio4 formed by SiO2, Al2O3 and a part of reduced FeO. Due to the existence of FeO in the slag, the melting point of the slag is reduced, and the slag has good fluidity. In the process of falling down (which is also the process of temperature rising), the FeO contained in the slag is gradually reduced and lost, while the content of Cao increases, and finally the final slag flows into the hearth.
When smelting with self fluxing ore, because the ore contains more Cao, and it can be in good contact with acidic SiO2, Cao immediately participates in the slagging reaction at the beginning of smelting, especially when smelting with self fluxing sinter, Cao forms slag with SiO2, Al2O3, etc. as early as in the sintering process, so the CaO content in the primary slag of this kind of ore is higher The composition of slag also changes little in the process of slag reduction.
Blast furnace products
The main products of blast furnace smelting are pig iron and ferroalloy, and the by-products are slag, gas and furnace dust.
pig iron
Pig iron is an iron carbon alloy with more than 2% carbon, which also contains Si, Mn, s, P and other impurities.
Pig iron can be divided into two categories according to its use and composition. One is steel-making pig iron: the carbon in the pig iron exists in the form of compound, and its cross section is silver white, also known as white iron; the other is casting pig iron: it is directly used to make machine parts.
ferroalloy
Iron and any kind of metal or nonmetal alloy are called ferroalloy (some are also called alloy pig iron). There are many kinds of ferroalloys, such as ferrosilicon, ferromanganese, ferrochrome, ferromolybdenum, ferrotungsten, etc.
Slag, gas and dust
Slag, gas and dust are by-products of blast furnace. They were discarded as waste before, but now they are widely used in building materials.









