Erosion-Wear Experimental Materials and Equipment
Experimental Materials
Tungsten carbide (WC) carbide?is a composite material produced using powder metallurgy techniques, with WC, a metal carbide that is difficult to dissolve, as the matrix and a binder added. It is characterized by high hardness and strong wear resistance. The WC carbide?used in this experiment is YG8, which is employed as the coating material for the valve core and outlet sleeve in coal direct liquefaction devices. YG8 is a tungsten-cobalt carbide?with a cobalt binder, a density of 14.6 g/cm3, a hardness of HV 1350, an elastic modulus of 540 MPa, a bending strength of 1500 MPa, and a compressive strength of 4470 MPa.
The experiment uses quartz sand (SiO?) particles as the erosion particles, which are commonly used in erosion-wear tests. The particles are sieved to achieve an average particle size of 150 μm, as shown in Figure 1.

Experimental Equipment
The experiment uses a self-made shock wave-driven gas-solid two-phase flow erosion-wear testing device. This device primarily consists of a shock wave generator, a velocity measurement system, a high-speed camera, a heating system, and a temperature control system.

In this setup, nitrogen gas is connected to the driving section to generate shock waves with a specific Mach number. The driving section and the driven section are separated by an aluminum film. Experimental particles are placed on a tin foil located between the driven section and the accelerating section. By adjusting the pressure relief valve, gas is introduced into the driving section of the shock tube. When the pressure difference across the film reaches a critical value, the film ruptures suddenly, generating a shock wave. The high-speed gas flow then propels the solid particles through the accelerating section to the desired experimental velocity. Upon impacting the specimen surface, the high-speed particles cause material loss, thus facilitating the erosion process.
The driving section, driven section, and accelerating section are each equipped with dynamic pressure sensors, charge amplifiers, and dynamic test analyzers to measure shock wave velocity. A high-speed camera in the experimental section captures the particle motion trajectories. The specimen holder is equipped with a temperature heating system and a temperature control system, allowing for adjustment to the required experimental temperature.
Erosion-Wear Experimental Parameters and Methods
Experimental Parameters
The impact angle, denoted as θ (see Figure 3), is defined as the angle between the axis of the shock tube and the surface of the specimen being eroded (0°to 90°). The desired impact angle is achieved by rotating the specimen holder.
The impact distance L is the distance between the center of the shock tube’s outlet and the center of the specimen’s surface. Typically, L is set between 30 and 50 mm during experiments. When conducting experiments at different impact angles, the position of the shock tube needs to be adjusted to maintain a consistent impact distance.

In the experiment, the impact velocity is adjusted by changing the thickness of the aluminum foil. Aluminum foils with thicknesses of 0.13 mm, 0.20 mm, and 0.30 mm are used for the erosion-wear tests. A high-speed camera is employed to record the particle trajectories. By analyzing these trajectories, the particle velocity v p is determined.
![]()
In the equation, ΔI represents the distance between the ends of the particle clusters in consecutive frames, measured in meters; Δn is the number of frames between measurements; and f is the filming frequency, measured in frames per second (FPS).
Using the high-speed camera, the velocity of 150 μm SiO? particles is tested. By replacing aluminum foils of different thicknesses, the corresponding membrane rupture pressure ratios are obtained, which in turn allows for the determination of the impact velocity. The velocities corresponding to different aluminum foil thicknesses are summarized in Table 1. The specific calculation method for particle velocity can be found in the referenced literature.

Experimental Method
Before the experiment, the nickel-based carbide specimens are first polished using 1000# sandpaper. The specimens are then cleaned with an ultrasonic cleaner, air-dried, and weighed to obtain an average value. The specimens are fixed onto the specimen holder, and the angle of the specimen and the distance between the shock tube and the specimen are adjusted. The temperature control system is activated, and the experimental temperature is set. Aluminum foils of the appropriate thickness are selected and solid particles are loaded simultaneously.
The lighting is turned on, and the dynamic testing analyzer and high-speed camera are activated. The camera lens height is adjusted so that the distance from the shock tube outlet to the specimen surface is within the field of view of the high-speed camera. The nitrogen gas valve is then opened to start the experiment. When the aluminum foil ruptures, the valve is immediately closed, and the high-speed camera captures the particle trajectories during the experiment.
At the end of the experiment, the specimen is cooled, cleaned, and dried. The specimen is weighed 10 times using an electronic balance to record the average weight. The erosion-wear rate is then calculated using equation (2).
![]()
In the equation, E represents the erosion-wear rate, measured in mg/g; Δm is the mass loss of the material, measured in mg; and m p is the mass of a single impact particle, measured in grams.
Analysis and Discussion of Erosion-Wear Experimental Results
Effect of Impact Angle on YG8 Wear Rate
Under an impact velocity of 175 m/s, the erosion-wear rates of the specimens were measured by varying the impact angles, as shown in Figure 4.?
From Figure 4, it can be observed that the erosion-wear rate of the specimen initially increases and then decreases as the impact angle increases. The erosion-wear rate reaches its peak at an impact angle of 75°. The experimental results indicate that YG8 is a typical brittle material, with the maximum erosion-wear rate occurring at high impact angles. The erosion-wear characteristics of YG8 are consistent with the behavior of brittle materials, where the erosion-wear rate varies with the impact angle.
Impact Angle on Coating Erosion-Wear Performance
Particle velocity is a crucial factor affecting the wear rate of materials. Impact experiments were conducted on specimens at impact angles of 30°, 60°, and 90° under three different impact velocities: 148 m/s, 175 m/s, and 200 m/s. The relationship between erosion-wear rate and particle impact velocity is shown.
Figure 5 demonstrates that, at all three impact angles, the erosion-wear rate of the material increases with increasing impact velocity. There is a critical impact velocity at which erosion-wear begins, related to the abrasive properties and the material’s characteristics. Erosion-wear occurs only when the velocity exceeds this critical value. Extensive erosion tests indicate that the erosion-wear rate has the following relationship with particle velocity:
E=kv” (3)
where
v is the particle velocity in m/s;
k is a constant; and
n is the velocity index. A higher
n value indicates that the erosion-wear rate of the material is more influenced by the particle impact velocity.
Fitting the experimental data to equation (3) yields velocity indices of 2.34, 2.27, and 2.28 for impact angles of 30°, 60°, and 90°, respectively, for YG8 material.
Analysis of Erosion-Wear Mechanisms
Analysis of the erosion-wear morphology of specimens at an impact angle of 90° reveals that the erosion-wear is primarily driven by the impact forces of the solid particles directly striking the composite layer. Due to the high brittleness of both tungsten carbide particles and the composite layer matrix, high-velocity solid particle impacts more readily induce plastic deformation or crack formation, leading to the development of pits and cracks.
During the erosion process, the abrasive quartz sand continuously impacts the surface, creating numerous pits. The edges of these pits accumulate material that has been deformed and squeezed out, forming a lip-like flange. With continued particle impacts, this flange is progressively eroded and stripped away due to repeated compression. The wear mechanism can be summarized as erosion-induced compression leading to pit formation and material detachment.
As the reinforcement phase, WC particles have much higher hardness and stiffness compared to quartz sand, which helps them better withstand the abrasive impacts. During the erosion-wear process, the coating undergoes cutting and plowing effects from the sharp edges of the abrasive particles, resulting in plastic deformation, progressive fatigue, and delamination. The protruding WC particles bear the brunt of the abrasive impact. Table 2 shows the elemental chemical composition of the specimen surface before and after the erosion experiments.
Conclusion
As the impact angle increases, the erosion-wear rate of YG8 material first increases and then decreases, reaching a maximum at an impact angle of 75°. YG8 exhibits the erosion-wear characteristics typical of brittle materials.
At impact angles of 30°, 60°, and 90°, the erosion-wear rate of YG8 material increases with rising impact velocity. The corresponding velocity indices, obtained from fitting the erosion-wear rate versus velocity relationship, are 2.34, 2.27, and 2.28, respectively.
The primary erosion-wear mechanism for YG8 material involves the formation of pits and microcracks on the material surface due to high-angle impacts. These features are caused by the detachment of Co and WC particles from the matrix and the development of microcracks under high-velocity impacts.
]]>
Experimental Methods
Raw Materials
The experiment uses coarse and extra-coarse WC powders from well-known suppliers, with their main characteristics shown in Table 1. Additionally, 2.0 μm cobalt powder from the same supplier was also used.

Experimental Methods
For the preparation of WC-10%Co (where all content is given in weight percentage), weigh 900 g of WC, 100 g of Co, and 20 g of PEG. Measure 235 mL of alcohol and 2000 g of grinding balls. Add these into a 2.4 L ball mill. The mill is operated at a speed of 63 r/min for 14.5 hours. After milling, the mixture is dried, sieved, and then pressed into samples weighing 10 g each. The samples are sintered in a continuous vacuum sintering furnace at 1450°C.
Particle Size Measurement
For coarse tungsten carbide, measure the Fischer particle size in both the as-supplied and milled states. The samples are resin-mounted and analyzed using a metallurgical microscope to determine the grain size and particle size distribution of the powder. The alloy grain size and particle size distribution are measured using classic metallographic methods, and the coercive force of the samples is also assessed.
Results and Analysis
Fischer Particle Size (Fsss) and Alloy Grain Size
As-Supplied Particle Size and Alloy Grain Size
The metallographic images of alloys made from WC powders #1 and #2 are shown in Figures 1 and 2, respectively. Comparing Figures 1 and 2, it can be observed that the WC grain size in Figure 2 appears to be slightly coarser than in Figure 1. This indicates that coarser as-supplied Fsss particle sizes of WC lead to coarser grain sizes in the WC-Co alloys. Metallographic analysis shows that the average WC grain sizes for alloys made from powders #1 and #2 are 4.8 μm and 5.8 μm, respectively. Thus, the average grain size of WC in sample #2 is 1.2 times that in sample #1. The as-supplied Fsss particle size of #2 WC powder is 2.5 times that of #1 WC powder. Clearly, there is no direct proportional relationship between the as-supplied Fsss particle size of WC powder and the alloy grain size. Additionally, the Fsss particle size values for #1 WC powder are 2.5 times the alloy grain size, and for #2 WC powder, it is 5.3 times the alloy grain size. This indicates that the as-supplied WC powders for both samples are primarily aggregated polycrystalline WC particles, with more severe agglomeration for coarser WC powders.


Milled Particle Size and Alloy Grain Size
Comparing Table 1 with the metallographic grain sizes in Figures 1 and 2, it can be seen that the Fsss particle sizes of milled WC powders #1 and #2 are relatively close to the alloy grain sizes. Moreover, the measured alloy grain sizes are higher than the milled Fsss particle size values. This discrepancy is due to differences in measurement principles as well as grain growth during the sintering process. However, it clearly indicates that the Fsss particle sizes of coarse WC powders in the milled state are very close to the alloy grain sizes. The ratios of average grain sizes to milled particle sizes for alloys #1 and #2 are 1.15 and 1.31, respectively.
Raw Material WC Grain Size and Alloy Grain Size
Results from Direct Metallographic Measurement
Metallographic images of #1 and #2 WC powders after mounting and etching are shown in Figures 3 and 4. The grain sizes measured using metallographic methods are 5.31 μm and 8.5 μm, respectively. The grain size distributions of the powders and alloys are shown in Figures 5 and 6.


Figures 3 and 4 clearly indicate that the grain size of #2 WC is significantly larger than that of #1 WC. This suggests that WC with a coarser as-supplied Fischer particle size also has coarser grains. Additionally, it is evident that #1 WC exhibits better dispersion, with less pronounced sintering between particles compared to #2 WC. The severe sintering in #2 WC particles is a major reason why the metallographic grain size is much larger than the alloy grain size, and also explains why the grains in #2 WC are much larger than those in #1 WC.
From the grain size distribution of the raw powders and alloys in Figures 5 and 6, it can be seen that sample #1 contains coarse WC grains of 15–20 μm in the raw material, which are not present in the alloy. In contrast, sample #2 has a substantial amount of WC grains in the 15–35 μm range, though only a small amount of 15–20 μm grains are found in the alloy. This suggests that the severe sintering of the mounted WC, although difficult to distinguish by metallographic methods after etching, was fragmented during the intense grinding process.
Moreover, comparing the WC and alloy grain size distributions in Figures 5 and 6 shows that the grain size distribution of WC in sample #1 is more consistent with the alloy grain distribution than in sample #2. This consistency is a significant reason why many researchers believe that WC similar to sample #1 is more conducive to producing coarse alloys with a more uniform grain size.
WC Particle Size and Alloy Coercive Force
The coercive forces of the alloys made from #1 and #2 powders are 4.6 kA/m and 4.3 kA/m, respectively. The relationship between the WC-Co alloy grain size and the alloy’s coercive force can be expressed using the empirical formula (1).

In the formula:
- Hc= coercive force of the alloy (kA/m)
- Com= cobalt content in the alloy (%)
- Dwc= average WC grain size in the alloy (μm)
According to the calculations, the average grain sizes of alloys #1 and #2 are 7.4 μm and 8.8 μm, respectively. Clearly, the calculated grain sizes are significantly larger than the measured grain sizes, but the difference between the average grain sizes of alloys #2 and #1 is close to the difference observed using metallographic methods. The results obtained from formula (1) do not show a clear quantitative relationship with the Fsss particle sizes of the raw WC in both states, but the size of the raw material particles can still be used to predict the alloy grain size and coercive force.
Conclusions
Based on the above, the following conclusions can be drawn:
1.Coarse WC powders with larger as-supplied Fsss particle sizes tend to have higher milled Fsss particle sizes and larger grain sizes, leading to alloys with larger grain sizes.
2.The Fsss particle size in the milled state of coarse WC can be used to evaluate the grain size of coarse WC and predict the grain size of WC-Co alloys. Under the test conditions, the alloy grain size is 1.1 to 1.3 times the Fsss particle size of the milled WC.
3.Coarse WC powders with as-supplied Fsss particle sizes around 10 μm have a better consistency in grain size distribution with the alloy WC grain size distribution compared to extremely coarse WC powders with Fsss particle sizes above 25 μm.
]]>
Experimental Materials and Methods
Tungsten carbide (WC) powders were used as the raw material. Four different particle sizes of WC powder were selected: 4.0 μm, 2.2 μm, 1.1 μm, and 0.5 μm, which were labeled as WC1, WC2, WC3, and WC4, respectively. Metal cobalt (Co) powder was used as the binder phase, and paraffin was used as the forming agent. Four different WC-Co mixtures with varying particle sizes were prepared.
The mixtures were processed into green bodies with specific shapes and densities using extrusion forming equipment. The pressed green bodies were then placed in a sintering furnace and subjected to high-temperature sintering at 1400°C for 30 minutes, followed by cooling, to form cemented carbide bars.
The magnetic properties of the cemented carbide bars were tested using a magnetic performance tester, measuring parameters such as coercive force (Hc) and saturation magnetization (Bs), and the results were analyzed.
Experimental Results and Analysis

Effect of Particle Size on Coercive Force
As shown in Table 1, the coercive force of cemented carbide bars increases with decreasing WC particle size, while the saturation magnetization also increases. This indicates that cemented carbide bars made with fine and ultrafine WC particles exhibit poorer magnetic properties. Among the samples, the ultrafine WC (WC4) shows the highest coercive force of 4450 A/m, followed by medium-sized WC (WC3) with a coercive force of 3300 A/m. Coarse WC (WC2) and very coarse WC (WC1) have lower coercive forces, at 2350 A/m and 1200 A/m, respectively. The increase in coercive force with decreasing WC particle size is primarily due to the increase in internal defects and dislocations within smaller particles. These defects and dislocations create resistance to domain wall movement, making the magnetization process more difficult and requiring a larger external magnetic field to achieve saturation, thereby increasing the coercive force.
Effect of Particle Size on Material Magnetic Performance Stability
For fine and ultrafine WC particles, the larger grain boundary area facilitates grain boundary diffusion and reactions, which reduces the material’s magnetic properties. As the WC particle size decreases, the magnetic saturation of cemented carbide bars gradually increases. Specifically: coarse WC (WC1) exhibits the lowest magnetic saturation at only 1.25 T; medium-sized WC (WC2) has a magnetic saturation of 1.15 T; fine WC (WC3) and ultrafine WC (WC4) show higher magnetic saturations at 1.05 T and 0.93 T, respectively. This is likely because fine and ultrafine WC particles have higher chemical reactivity, promoting the diffusion and bonding of the Co binder, thereby improving the stability of the material’s magnetic performance.
Magnetic saturation is an indicator of the remaining proportion of magnetizable material and is closely related to magnetic properties such as coercive force and remanence. The impact of WC particle size on magnetic saturation can be attributed to the degree of solubility of the binder phase in the cemented carbide bars. Coarse and medium-sized WC particles, having larger specific surface areas, have more contact with the Co binder, which enhances the solubility of Co in the cemented carbide bars. This effectively improves the material’s magnetic performance stability, resulting in higher coercive force and better magnetic stability. Conversely, fine and ultrafine WC particles, with smaller specific surface areas, reduce the effectiveness of the Co binder, potentially affecting the material’s hardness and magnetic properties. Thus, selecting the appropriate particle size during the preparation of cemented carbide bars is crucial for achieving the best overall performance based on specific application needs.
Impact of Gamma Phase on Material Performance
For cemented carbide materials, the proportion of the gamma phase directly affects the material’s hardness and magnetic properties. Variations in carbon and oxygen content also influence the gamma phase proportion and must be considered during material preparation. Generally, higher carbon content leads to an increase in the gamma phase proportion, thereby enhancing the material’s hardness and magnetic performance. Therefore, different WC particle sizes may have varying carbon and oxygen contents, which also affects the gamma phase proportion and the overall performance of the material.
Discussion on Sintering Atmosphere
In the sintering process of cemented carbides, the choice and control of the atmosphere have a decisive impact on the final microstructure and magnetic properties of the material. The atmosphere not only affects the chemical reactions during sintering but also directly relates to the microstructure and final performance of the cemented carbide. The types of sintering atmospheres are as follows:
Oxidizing Atmosphere:? air.
Reducing Atmosphere: Contains components such as H? or CO: hydrogen atmosphere for cemented carbide sintering.
Inert or Neutral Atmosphere: Argon, helium, vacuum.
Carburizing Atmosphere: Contains high components that cause carburization of the sintered body, such as CO, methane, and hydrocarbon gases.
Nitrogen-Based Atmosphere: High nitrogen content sintering atmosphere: 10% H? in N?.
We mainly selected vacuum, argon, and hydrogen atmospheres for discussion. The variations in coercive force and magnetic saturation of cemented carbides sintered in argon, vacuum, and hydrogen atmospheres differ depending on the atmosphere, as shown in Table 2.

From Table 2, it can be observed that under vacuum and argon atmospheres, the coercive force (Hc) of cemented carbide bar is higher compared to that in a hydrogen atmosphere. Conversely, the saturation magnetization (Bs) is lowest in a hydrogen atmosphere compared to vacuum and argon atmospheres.
Under vacuum and argon atmospheres, the effective control of oxygen partial pressure and the exclusion of volatile elements result in fewer pores and inclusions, clearer grain boundaries, and better grain growth, thereby enhancing the magnetic properties of the material. In contrast, in a hydrogen atmosphere, the reducing nature of hydrogen may reduce some elements in the cemented carbide, leading to the presence of uncertain phase components, poor grain growth, and subsequently affecting the material’s magnetic properties.
For coercive force (Hc), it is largely dependent on the material’s microstructure and magnetic anisotropy. Under vacuum and argon atmospheres, effective control of oxygen partial pressure and exclusion of volatile elements reduce magnetic anisotropy in the cemented carbide, which improves coercive force. However, in a hydrogen atmosphere, hydrogen’s reducing effect can lead to the reduction of some elements in the cemented carbide, resulting in grain defects and inclusions that directly affect magnetic anisotropy and reduce coercive force.
Regarding saturation magnetization (Bs), the relative magnetic saturation value in cemented carbide is influenced by factors affecting carbon content in the alloy. In vacuum or argon atmospheres, effective control of oxygen content reduces carbon loss. Although the pressed green body contains oxygen, which can be reduced by free carbon and carbon in WC (MeO + C = Me + CO), the oxygen content in these atmospheres is relatively low. In a hydrogen atmosphere, decarburization reactions (WC + 2H? → CH? + C) begin at around 100°C. Throughout the preparation process, the material is exposed to a decarburizing atmosphere, leading to a lower relative magnetic saturation value.

Conclusion
This experiment investigated the effects of different particle sizes and sintering atmospheres on the magnetic properties of cemented carbide bars. By comparing the magnetic properties of cemented carbide under different WC particle sizes (coarse, medium, fine, and ultrafine) and sintering atmospheres (vacuum, argon, and hydrogen), it was found that both particle size and atmosphere have a significant impact on the magnetic performance of the material.
From the perspective of particle size, as the WC particle size decreases, the coercive force of the cemented carbide bars increases, while magnetic saturation also increases. This indicates that particle size has a substantial effect on the magnetic properties of cemented carbide. Fine and ultrafine WC particles, due to their higher chemical reactivity and good sintering performance, can promote the diffusion and bonding of the Co binder, thus enhancing the stability of the material’s magnetic performance. However, smaller particle sizes may lead to increased porosity and inclusions, affecting the material’s hardness and magnetic performance. Therefore, the choice of particle size should be tailored to the specific application needs when preparing cemented carbide.
Regarding the atmosphere, cemented carbide bars sintered under vacuum and argon atmospheres exhibited higher coercive force and better magnetic stability. This is because these atmospheres effectively control the oxygen content and volatile elements, reducing porosity and inclusions, and promoting clearer grain boundaries and grain growth. In contrast, cemented carbide bars sintered in a hydrogen atmosphere showed significantly lower magnetic saturation. This is likely due to the decarburizing effect of hydrogen. Therefore, selecting the appropriate sintering atmosphere is crucial for obtaining cemented carbide bars with excellent magnetic properties. Further improvements in cemented carbide performance can be achieved by optimizing sintering process parameters and adding suppressants.
]]>
The Effect of Cobalt Content

Figure 3 shows the microstructure after oxidation of carbides?with different cobalt contents (all WC materials are WC-1). As the cobalt content increases, the microstructure of the carbide?oxides changes significantly. The oxide of the WC-6%Co carbide?has more and larger pores, the pores in the oxide of the WC-10%Co carbide?are significantly reduced, and the oxide of the WC-14%Co carbide?has virtually no large pores.

Figure 4 shows the oxidation weight gain curves of carbides?with different cobalt contents. As the cobalt content increases, the oxidation weight gain of the carbides decreases sequentially. At 900°C, the oxidation weight gain of WC-6%Co, WC-10%Co, and WC-14%Co carbides are 11.92%, 11.46%, and 11.26%, respectively. Compared to WC-6%Co carbide, the oxidation weight gain of WC-10%Co and WC-14%Co carbides?at 900°C decreased by 3.8% and 5.5%, respectively. Therefore, although increasing the Co content can improve the high-temperature oxidation resistance of carbides, the improvement is not significant.

Table 3 lists the oxidation reaction equations of each component in the carbide?and their Gibbs free energy. It is well known that during the oxidation of carbides, the oxidation of WC to WO3 results in significant volume expansion. The oxide WO3 is loose, porous, and volatile, producing volatile gases such as CO2, which provide more pathways for the oxidation diffusion process, thereby exacerbating the oxidation of the carbide. Although the binder phase is more prone to oxidation than the hard phase, the oxide formed from the binder phase is the relatively dense CoWO4, which can slow down the oxidation diffusion process of the carbide. Therefore, with the increase in cobalt content, more CoWO4 and less WO3 are formed, resulting in a denser microstructure of the oxides and consequently improving the high-temperature oxidation resistance of the carbide.

Table 4 shows the room temperature hardness and high-temperature hardness of carbides?with different cobalt contents. At room temperature, the more cobalt content, the lower the hardness of the carbide. When the temperature rises to 800°C, the hardness of the carbides decreases significantly, with the rate of decrease reducing as the cobalt content increases. At 800°C, the hardness of carbides with higher cobalt content is actually higher than that of carbides with lower cobalt content.
Both the hard phase and the binder phase exhibit some thermal expansion at high temperatures, with the binder phase experiencing greater thermal expansion and generating larger stress, which offsets some of the load force. This is one of the reasons why the high-temperature hardness of the carbide?increases with the increase in cobalt content.
The Effect of WC Grain Size

Figure 6 shows the oxidation weight gain curves of 4#, 5#, and 6# carbides?prepared with WC of different Fischer particle sizes. From room temperature to 825°C, the oxidation weight gain curves of the three carbides with different WC grain sizes overlap; however, in the range of 825-900°C, the finer the WC grains, the less the oxidation weight gain of the carbides. At 900°C, the oxidation weight gains of 4#, 5#, and 6# carbides?are 9.18%, 8.67%, and 8.20%, respectively. Compared to the 4# carbide, the oxidation weight gain of the 5# and 6# carbides?at 900°C decreased by 5.6% and 10.7%, respectively. Therefore, under the same Co content, refining the WC grains can improve the high-temperature oxidation resistance of carbides.

Figure 7 shows the XRD diffraction patterns after oxidation of carbides?with different WC grain sizes. Since the compositions of 4#, 5#, and 6# carbides?are the same, there is no significant difference in their oxidation products. Therefore, the diffraction patterns of the oxides of the three carbides?with different WC grain sizes are essentially identical.
The Oxidation Resistance and hardness Differences of Carbides with Different WC Grain Sizes
The differences in the oxidation resistance of carbides?with different WC grain sizes can be mainly attributed to the following two points:
In the case of a uniform carbide?structure, finer WC grains result in more phase boundaries between WC and the binder phase. The finer WC grains are better encapsulated by the binder phase, and the oxidation products of the binder phase can, to some extent, hinder the oxidation diffusion process, thereby improving the high-temperature oxidation resistance of the carbide.
Finer WC grains have fewer grain boundary defects and smaller grain boundary voids between the WC grains, which correspondingly reduce the oxidation diffusion channels, thus enhancing the high-temperature oxidation performance of the carbide.

Table 5 shows the room temperature hardness and high-temperature hardness of carbides?with different WC grain sizes. At room temperature, the finer the WC grains, the higher the hardness of the carbide. When the temperature rises to 800°C, the hardness of the carbides decreases significantly, and the rate of decrease in high-temperature hardness increases as the WC grain size decreases. Clearly, although the room temperature hardness of the carbide?increases as the WC grain size decreases, the high-temperature hardness becomes lower.
The Effect of TaC/NbC/TiC Additives

Figure 8 shows the oxidation weight gain curves of carbides?with different carbide additives (all WC materials are WC-3). The oxidation weight gain curves and oxide diffraction patterns of WC-Co and WC-Co-TaC carbides?are basically the same, with oxidation weight gains of 10.58% and 10.20% at 900°C, respectively. Among the four carbides, WC-Co-NbC carbide?has the highest oxidation weight gain, while WC-Co-TiC carbide?has the lowest oxidation weight gain, with oxidation weight gains of 11.68% and 9.05% at 900°C, respectively.

Figure 9 shows the XRD diffraction patterns of carbides?with different carbide additives after oxidation. The oxidation of the carbides produces corresponding oxides.
In WC-Co carbides, the added TaC, NbC, and TiC all exist in the form of W-containing solid solutions. The (Nb,W)C solid solution oxidizes earlier than WC and has many phase boundaries with WC. Without the protective “encapsulation” of the binder phase, the oxidation of the solid solution promotes the oxidation of WC, thereby accelerating the oxidation of the carbide. The oxidation weight gain of WC-Co-TaC carbide?is the same as that of WC-Co carbide. This is because the (Ta,W)C solid solution reacts simultaneously with WC, and since the hard phase WC is the main component, the loose and porous WO3 phase predominantly controls the oxidation rate of the carbide. Therefore, the addition of TaC does not significantly affect the high-temperature oxidation resistance of the carbide.
In summary, under the same conditions of grain size and cobalt content, the addition of TaC has no significant effect on the high-temperature oxidation resistance of the carbide. However, the addition of NbC significantly reduces the high-temperature oxidation resistance of the carbide, with a reduction of 10.4%, while the addition of TiC significantly improves the high-temperature oxidation resistance of the carbide, with an improvement of 14.5%.

Table 6 shows the room temperature hardness and high-temperature hardness of carbides?with different carbide additives. At room temperature, the hardness of the carbides with TaC, NbC, and TiC additives is comparable to that of the WC-Co carbide. When the temperature rises to 800°C, the high-temperature hardness of the carbides with TaC, NbC, and TiC additives is higher than that of the WC-Co carbide, and the rate of decrease in high-temperature hardness is significantly reduced.
It is well known that solid solutions exhibit good red hardness and provide structural support to the overall carbide, helping it maintain high hardness under high-temperature conditions. Additionally, the solid solutions contribute to solid solution strengthening of the Co phase, which increases the hardness of the Co phase. Therefore, the addition of TaC, NbC, and TiC results in carbides?exhibiting good high-temperature hardness.
Conclusion
This study investigated the effects of cobalt content, WC grain size, and types of solid solutions on the high-temperature oxidation resistance and high-temperature hardness of carbides. The conclusions are as follows:
1.Increasing the cobalt content improves the high-temperature oxidation resistance of the carbide?and significantly increases the high-temperature hardness.
2.Reducing the WC grain size enhances the high-temperature oxidation resistance of the carbide?but significantly reduces the high-temperature hardness.
3.Compared to WC-Co carbides, the addition of TaC has no significant effect on the high-temperature oxidation resistance of the carbide, the addition of NbC decreases the high-temperature oxidation resistance, and the addition of TiC significantly improves the high-temperature oxidation resistance. All three additives, TaC, NbC, and TiC, significantly enhance the high-temperature hardness of the carbide.
]]>Research status
For WC-Co carbide, the rapidly advancing Powder Bed Fusion (PBF) additive manufacturing (AM) technology has shown unique advantages in producing complex structures of metal parts made of carbide. However, when manufacturing WC-Co carbide with high melting points and high content of hard phases, issues such as difficult-to-eliminate cracks, pores, abnormal grain growth, oxidation decarburization, and brittleness often arise, leading to poor mechanical properties of the produced carbide. In recent years, there have been many reports on the use of Green Additive Manufacturing-Debinding and Sintering (GAM-DS) technology to fabricate WC-Co carbide, which have shown significant advantages in addressing issues such as cracking, abnormal grain growth, oxidation decarburization, and brittleness in PBF carbide. However, the process of preparing green bodies is prone to defects such as pores, interlayer cracks, uneven carbon distribution, and weak local bonding, resulting in problems such as porosity, uneven sintering shrinkage, and uneven microstructure in the sintered bodies. Compared with powder metallurgy, the prepared carbide have relatively low relative densities, and there is a significant gap in mechanical properties.
Brief introduction of research results
Recently, the State Key Laboratory of Powder Metallurgy at Central South University has employed Material Extrusion Additive Manufacturing (MEX) – Debinding and Sintering (DS) technology to successfully produce high-strength and tough WC-9Co cemented carbide with no pores, no cracks, and uniform shrinkage in all directions. Its relative density is approximately 99.7%, and its Vickers hardness, transverse fracture strength, and fracture toughness reach 1525±3HV30, 3492±45MPa, and 20.4±0.5 MPa·m1/2 respectively. The comprehensive mechanical properties are comparable to those of high-performance WC-Co carbide prepared by powder metallurgy processes. The relevant work, titled “Material extrusion additive manufacturing of WC-9Co cemented carbide,” was published in the top international journal “Additive Manufacturing.”
research chart

FIG. 1 Microstructure of MEX WC-9Co cemented carbide green

FIG. 2 Schematic diagram of stack pore formation of cemented carbide printing green billet: a. MEX stack pore formation; b. Increasing the overlap rate of microfilaments is conducive to reducing the stack porosity of green billet;

FIG. 3 Microstructure of MEX-DSWC-9Co cemented carbide

Figure 4 Micro-CT analysis results of internal defects in MEX-DS WC-9Co cemented carbide

Figure 5 Microstructure of WC-9Co cemented carbide: (a) MEX-DS; (b) Press forming – degreasing sintering

Figure 6 MEX-DS WC-Co carbide Co pool and Co rich zone

Figure 7 Transverse fracture strength and fracture toughness of WC-(8-12)Co cemented carbide prepared by different processes
Summary
Conclusion of the Paper
(1) By calculating the plasticity index of the printed feedstock with a powder loading of 54 Vol.%, the mechanism of green body printing defects was analyzed, and the green body MEX parameters were optimized. Using optimized parameters such as a printing temperature of 150°C, filament overlap rate of 30%, and printing layer thickness of 0.1mm, defect-free green bodies of WC-9Co cemented carbide with a relative density of 98.5% were prepared.
(2) Both excessively high or low temperatures during the debinding process using n-heptane can lead to debinding cracks. Rapid solvent evaporation during the drying process of debound bodies can also result in microcracks. By employing a two-step solvent debinding process, namely, n-heptane debinding at 30°C for 12 hours followed by kerosene debinding at 30°C for 1 hour, the solvent evaporation rate was reduced, resulting in high-quality debound bodies with no noticeable debinding defects and uniform distribution of binder.
(3) Defects in MEX green bodies can lead to the formation of Co-rich regions or pools, abnormal WC grains, residual pores, etc., in WC-Co carbide. These defects can be improved or eliminated during the sintering process through liquid phase flow and rearrangement of WC particles. By optimizing the MEX green body printing and solvent debinding processes to eliminate printing and debinding defects, it is possible to eliminate defects such as sintering pores, cracks, Co pools, abnormal grain growth, etc., in WC-Co carbide, resulting in near-full-density WC-9Co carbide.
(4) By employing MEX green bodies, a two-step solvent debinding process, and a continuous thermal debinding-vacuum pressure sintering process, WC-9Co carbide with uniform microstructure, smaller grain size, and relatively uniform distribution were prepared. The Vickers hardness, transverse fracture strength, and fracture toughness were measured to be 1525±3HV30, 3492±45MPa, and 20.4±0.5MPa·m1/2, respectively. The comprehensive mechanical properties were superior to those reported by recent additive manufacturing technologies and comparable to those of WC-Co carbide prepared by traditional powder metallurgy processes.
Main Innovations of the paper of WC-Co carbide additive manufacturing
The use of WC-Co carbide MEX-DS technology to prepare near-full-density WC-9Co carbide, with a transverse fracture strength reaching 3492MPa and a fracture toughness exceeding 20MPa·m1/2, has significantly improved the transverse fracture strength of WC-Co carbide prepared by current AM methods (ranging from 1500-2000 MPa to 3000-4000MPa with HIP treatment) and increased fracture toughness to above 20MPa·m1/2. The comprehensive mechanical properties are significantly better than those reported by similar studies and comparable to similar products prepared by powder metallurgy. The research results are of great significance for addressing the challenging issues of porosity, cracks, and harmful phases encountered in current carbide additive manufacturing and for the development of carbide additive manufacturing technology.
]]>
Powder Extrusion Molding (PEM)
Powder extrusion molding (PEM) involves the extrusion of a mixture of powder, binders, plasticizers, etc., through a die nozzle to obtain the desired shape and size of the blank. The basic process of PEM includes powder mixing, granulation, extrusion molding, debinding, and sintering. PEM can be operated at low temperature and low pressure, with no limitation on the length of the product, uniform longitudinal density, and advantages such as strong forming continuity, low cost, and high efficiency, making it the main method of forming carbide?round bars today. Additionally, the use of hot extrusion molding has been adopted to prepare dispersion-strengthened materials and high-temperature alloys.
Powder Injection Molding (PIM)
Powder injection molding (PIM) combines traditional plastic molding processes with powder metallurgy technology. It involves mixing powder and molding agents, granulating, heating in an injection molding machine to form a flowable material, injecting into the mold cavity under pressure, and obtaining preformed blanks with uniform structure and complex geometry. Products produced by this method have good surface finish and shapes close to the final product. PIM improves the sintering performance by maintaining good flowability of the carbide rod powder during injection and enhancing the interaction between binders and alloy powders. Compared to traditional molding methods like die pressing, PIM offers advantages such as unrestricted product shapes, uniform product density, wide applicability, and consistent shrinkage of product parts, allowing better control of dimensional tolerances.

Cold Isostatic Pressing (CIP)
Cold isostatic pressing (CIP) involves placing carbide rod powder in a closed liquid environment at room temperature to form the powder under ultra-high pressure transmitted by the liquid. The pressure transmitted by the liquid medium during isostatic pressing is equal in all directions, resulting in uniform stress on the blank and significantly improved product performance. Typically, preforms are obtained through metal die pressing followed by cold isostatic pressing. After sintering, carbides produced by cold isostatic pressing exhibit low shrinkage, high density, and hardness.
Explosive Forming (EF)
Explosive forming is a special method of compacting blanks using intense explosive pressure. It involves placing explosive substances around a shell containing superhard powder. The enormous pressure generated during the explosion (up to 10 MPa) can rapidly compact blanks with very high relative density. Experiments have shown that the density of WC-8Co composite powder can reach 99.2% using explosive forming.
High Velocity Compaction (HVC)
High velocity compaction (HVC) involves compacting powder at pressures of 600 to 1000 MPa and velocities of 2 to 30 m/s using hydraulic-controlled heavy hammers. Compared to traditional die pressing, HVC achieves significantly faster compaction rates, up to 500 to 1000 times faster. This technology improves material properties, and the repeated compaction characteristic enables small to medium-sized equipment to produce large-sized components.

Warm Compaction (PM)
Warm compaction involves pressing carbide rod powder at temperatures of 100 to 150°C in heated molds to obtain blanks. The process effectively increases the density and strength of the pressed blanks. Recently, flow-assisted warm compaction (WFC) has been developed based on warm compaction, which has the capability to manufacture high-density parts with complex geometries, offering broad prospects for the manufacturing of parts with complex shapes such as side cavities and threaded holes.
Extrusion molding of carbide double helix hole bars
The so-called inner helical hole bar refers to the part of the spiral cutting tool that forms spiral cooling holes. When the tool is in operation, the inner holes can pass coolant, reducing the processing temperature. The angles of the cooling holes and the cutting edge of the drill tool are synchronized, typically at 45°, 30°, and 15°; the most commonly used conventional tool spiral cooling hole angle is 30°.
External helix extrusion for helix hole carbide rod
External helix extrusion involves the use of grooves on the male mold to forcefully change the direction of the extruded fluid material, rotating it to extrude the billet. The manufacturing of the cooling hole core rod is synchronized with the rotation direction of the mold thread. The entire process requires complex mold design and is similar to ordinary extrusion methods, making it one of the most widely used extrusion techniques domestically and internationally.
Internal helix extrusion
Internal helix extrusion utilizes the special structure of a double helix extruder to extend the core rod to the extrusion screw, rotating the core rod by the screw to achieve the helical action. This process has simpler mold design but requires strict control over extrusion parameters: the extrusion speed of the fluid and the rotation speed of the core rod must maintain a fixed proportion; otherwise, the geometric parameters of the product may not meet requirements. The fluid motion during extrusion is similar to that of other rods.
Due to the difficulty of this technique,there are a handful of companies possessing the capability of production, among which Meetyou Carbide stands out. There is not much difference in the extrusion method between triple helix holes and double helix holes, only the design of the core rod in the mold differs.
]]>The State of Carbide Compact Formation
After the carbide?compact is formed, it exists in a porous state. During the wet grinding process, the shape of WC is subjected to strong impacts, resulting in increased surface energy and enhanced reactivity. The longer the contact time of the compact with air, the greater the degree of oxidation, requiring more carbon for reduction. With the theoretical carbon content of carbide?remaining at 6.128%, the ratio of oxygen atoms to carbon atoms is 12/16. Therefore, for every additional unit of oxygen, it will consume 3/4 of the carbon content. This leads to the formation of the η phase more easily after alloy sintering.
The Existence of Oxygen in Carbide Mixtures
The oxygen content in the carbide?mixture can be considered to exist in three forms: occluded oxygen, cobalt surface oxygen, and oxygen in WO2 or WO3. Since the oxygen content measured by chemical oxygen determination includes the total of these three types of oxygen, it is difficult to determine their respective proportions in production. Therefore, this poses challenges to production. Additionally, oxygen enrichment in the environment is ubiquitous, so it is essential to manage each process reasonably in actual production.
Occluded Oxygen
Exists in the interstices of the compact and on the surface of the compact and mixture; generally removed by vacuum evacuation at the beginning of sintering, so it does not affect alloy sintering.
Cobalt Surface Oxygen
Due to the high susceptibility of cobalt to oxidation at room temperature, oxidation intensifies with increasing temperature. After wet grinding and subsequent drying, a layer of oxide film forms on the cobalt surface; the longer the material or compact is stored before sintering, the higher the degree of cobalt oxidation. This portion of the oxide requires carbon for reduction; before the temperature reaches 600°C during sintering, reduction mainly relies on free carbon, and the remaining unreduced oxides must be reduced by combined carbon. This portion of oxygen is critical to the carbon-oxygen balance during alloy sintering and is difficult to control.
WO2 or WO3 Oxygen
Also known as compound oxygen; before the carbonization of WC, WO3 gradually transforms into WO2 and then into tungsten powder (W), followed by carbonization. Some oxides may remain incompletely reduced or partially oxidized due to storage time, from W → W2C → WC, and may persist even after completion. Alternatively, inadequate protection during storage may lead to oxidation. These oxide residues are referred to as compound oxygen; the reduction temperature generally occurs before 1000°C, but severe oxidation may delay reduction until 1200°C. This oxide residue consumes carbon significantly, narrowing the margin for carbon levels and making it difficult to control sintering carbon content, thereby complicating the achievement of sufficient liquid phase formation.
The Form of Carbon in carbide
The carbon content in carbide?mainly exists in three ways: WC stoichiometry, carbon increment from binder decomposition, and carbon infiltration from furnace gases.
Generally, WC is adjusted according to the theoretical carbon content of carbide; reasonable carbon adjustment is made based on small samples before wet grinding; in the wax process, the carbon content is adjusted by subtracting the amount of carbon infiltrated from furnace gases and adding the amount of carbon consumed by oxides. In the rubber process, one-third of the rubber weight should be subtracted.
Carbon Increment from Binder Decomposition
During debinding and sintering, whether using wax, PEG, or rubber, there is more or less decomposition; thus, carbide?can gain carbon, although the amount of carbon increase varies with different binders. Since wax mainly relies on evaporation, it is generally considered not to increase carbon content. On the other hand, rubber and PEG rely on decomposition, with rubber decomposition occurring at higher temperatures, resulting in more carbon increase.
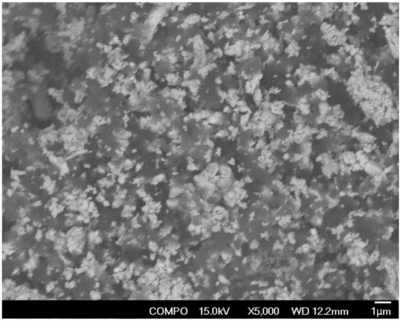
Carbon Infiltration from Furnace Gases
Since most heating elements, insulation layers, sintering plates or boats in carbide?sintering furnaces are made of graphite products, their effects become evident at 600°C; when sintering temperature rises above 1200°C, a large amount of carbon and CO released from graphite exacerbate carbon infiltration into carbide.
Impact of Cobalt on carbide?Properties
Cobalt has a hexagonal close-packed crystal structure, making it highly reactive and prone to oxidation. In WC-Co alloys, cobalt acts as the binder metal. When the cobalt phase exhibits the ε-Co crystal structure, with fewer slip planes (theoretically no more than 3), the alloy’s toughness is low. However, when the cobalt phase exhibits the α-Co crystal structure, the maximum number of theoretical slip planes can increase to 12, resulting in stronger fracture resistance. With increasing sintering temperature, the cobalt crystal structure shifts from hexagonal close-packed to face-centered cubic; the reverse occurs during cooling. Since tungsten dissolves more in cobalt, playing a “nailing” role, the transformation of crystal structure during cooling varies with the amount of tungsten dissolved.
Up to 1% of cobalt can dissolve in WC at room temperature; when the sintering temperature reaches between 400°C and 800°C, vigorous diffusion and rearrangement of cobalt occur. During this period, a lower amount of free carbon is more conducive to increased slip planes; this is advantageous in wax processes. However, rubber processes require completion of decomposition around 600°C, affecting the effective occurrence of cobalt phase slip planes.
At 1000°C during sintering, the oxide has almost completed the reduction process, so this stage is referred to as oxygen-free sintering. Carbon content in carbide?is generally tested at this stage; however, the so-called oxygen-free carbon contains only a minimal amount of oxygen. Nonetheless, oxide on the cobalt surface has been completely reduced by this point, and the edges of the cobalt phase have produced fewer liquid phases. At this stage, the compact has acquired some hardness, known as the pre-sintering stage. Products at this stage can undergo plastic processing if necessary.

Liquid Phase in Carbide
Theoretically, the liquid phase in WC-Co alloys appears at 1340°C. The temperature at which the liquid phase sufficiently appears varies with carbon content. As sintering temperature rises, the amount of liquid phase increases; fine WC particles gradually form a liquid phase. Intense shrinkage occurs in the product, reducing the distance between WC particles. Fine WC particles are gradually melted by larger particles, resulting in coarser WC particles. This phenomenon is known as grain growth. Grain growth during sintering is inevitable, particularly in ultrafine or submicron WC, where grain growth is more pronounced. To effectively inhibit excessive grain growth, inhibitors such as VC, TaC, and Cr3C2 can be added.
After sintering, undissolved WC and W2C rapidly precipitate, followed by ternary eutectic formation, laying the foundation for the alloy. The longer the cooling time above 1200°C, the more complete the precipitation, but the greater the opportunity for grain growth.

Conclusion
The pursuit of ternary eutectic structures is the most critical aspect of sintering in WC-Co carbide. Ternary eutectic structures form the fundamental framework of carbide. In the W-C-Co ternary system, effective handling of WC grain growth, allowing more tungsten to dissolve in cobalt without decarburization, thereby improving the durability and toughness of carbide, is always the goal of alloy manufacturers. A German technical expert once said: “The essence of sintering lies in ‘high temperature and low carbon’.”
]]>
Binder Jetting 3D printing technology has enabled the creation of even more complex structures, including carbide?tools with internal cooling channels.Binder Jetting Metal 3D Printing Technology
What is Binder Jetting?
Binder Jetting 3D printing technology combines material jetting and sintering processes to produce fully dense metal components. The lower cost of equipment also means significantly reduced part costs, and low-cost, high-volume parts are crucial for transitioning to production. Binder Jetting metal 3D printing technology has the potential to replace low-volume, high-cost metal injection molding and can also be used to produce complex and lightweight metal parts in other fields, such as gears or turbine impellers, greatly reducing 3D printing costs and shortening delivery times.

In Binder Jetting 3D printing process, ceramic hard material powder particles, including tungsten carbide particles, are bound together layer by layer using a bonding material containing cobalt, nickel, or iron. This bonding material not only serves as the binder between powder layers but also imparts excellent mechanical properties to the product and enables the production of fully dense parts. It can even selectively adjust the bending strength, toughness, and hardness. These 3D printed carbide?molds have greater geometric freedom than molds produced by traditional methods, allowing for the creation of more complex geometries.

Advantages of 3D Printing Compared to Traditional Machining Processes
Traditional machining processes typically involve compressing tungsten carbide powder uniformly in a flexible bag to manufacture large-sized carbide?components or carbide components with high aspect ratios (such as end mills and drill bit shanks). Although the production cycle of the compaction method is longer than that of molding methods, the manufacturing cost of the tool is lower, making this method more suitable for small-batch production.
carbide?components can also be formed by extrusion or injection molding. Extrusion processes are more suitable for the large-scale production of axially symmetric shaped components, while injection molding processes are typically used for the large-scale production of complex-shaped components. In both molding methods, the grade of tungsten carbide powder is suspended in organic binders, giving the tungsten carbide mixture a paste-like uniformity. The mixture is then extruded through holes or molded into cavities. The characteristics of the tungsten carbide powder grade determine the optimal ratio of powder to binder in the mixture and have a significant impact on the flow of the mixture through the extrusion or into the mold cavity.
After molding, compaction, extrusion, or injection molding of the components, it is necessary to remove the organic binder from the components before the final sintering stage. Sintering removes pores from the components, making them fully (or substantially) dense. During sintering, the metal bonds in the compacted shaped components become liquid, but the components can still maintain their shape due to the combined action of capillary forces and particle contacts.
After sintering, the geometric shape of the components remains unchanged, but the dimensions shrink. To obtain the desired component dimensions after sintering, shrinkage must be considered when designing the tool. When designing the tungsten carbide powder grades used to manufacture each tool, it must be ensured that the correct shrinkage rate is achieved when compressed under appropriate pressure.

Furthermore, combining differentiated metal powders with binder jetting and laser powder bed 3D printing technologies, along with manufacturing expertise in post-printing processes, can expedite the production of finished components and molds, thereby reducing downtime and enhancing performance.

Meetyou carbide??is also committed to flexible customized design and manufacturing of special metal and alloy components such as high-temperature alloys and refractory metals. Meanwhile, it is upgrading to become an outstanding 3D printing solution provider for high-density, large-sized, and scalable production of tungsten components.
]]>Aluminum alloy is a general term for alloys with aluminum as the base. The main alloying elements include Cu, Si, Mg, and Sn, while secondary elements may include nickel, titanium, chromium, lithium, and others. Aluminum alloys have low density, good plasticity for shaping and processing into various forms. They exhibit excellent electrical conductivity, thermal conductivity, and corrosion resistance. Alloys formed by adding specific elements not only maintain the lightweight properties of pure aluminum but also possess higher strength.

Classification of aluminum alloy
Aluminum alloys can be classified into deformed aluminum alloys and cast aluminum alloys based on their processing methods.
Deformed Aluminum Alloys: Deformed aluminum alloys can be further categorized into non-heat-treatable and heat-treatable alloys, both of which exhibit moderate strength and hardness. The challenge in machining lies in their high plasticity, resulting in the formation of built-up edge during cutting, making it difficult to achieve a satisfactory performance. Mechanical properties can be improved through heat treatment, but strengthening is mainly achieved through cold working deformation. This category includes high-purity aluminum, industrial high-purity aluminum, industrial pure aluminum, and corrosion-resistant aluminum.
Cast Aluminum Alloys: Cast aluminum alloys have low ductility, with elongation typically below 4%, making them unsuitable for pressure processing and mostly suitable for cutting operations. Silicon-aluminum alloys demonstrate good casting properties and excellent mechanical performance, making them the most widely used cast aluminum alloys. The machinability of silicon-aluminum alloys is influenced by the silicon content, with higher content leading to more severe tool wear and poorer machining performance. Mechanical properties of cast aluminum alloys can be enhanced through heat treatment methods such as quenching and aging. This category includes hard aluminum, forged aluminum, superhard aluminum, and special aluminum alloys.

Processing defects of aluminum alloy material
Insufficient Rigidity
Due to the strong toughness and resistance to bending of aluminum alloys, it also implies that aluminum alloys lack rigidity. In the machining of thin-walled aluminum alloy components, excessive machining pressure can lead to component deformation. During the cutting process, issues such as stretching, breaking, and surface squeezing may occur, causing displacement and resulting in irreversible situations for thin-walled aluminum alloy components.
Susceptible to Thermal Deformation
Compared to steel, the coefficient of expansion for aluminum alloys is typically 2.4 times that of steel. Therefore, significant heat energy is generated during the machining process, leading to thermal deformation issues in aluminum alloys.
Insufficient Hardness of Aluminum Alloy
During mechanical machining, scratching issues often arise, leading to a lack of glossiness on the surface of thin-walled aluminum alloy components, which does not meet machining standards. Besides daily operational issues, this problem is mainly attributed to the insufficient hardness of aluminum alloy materials.
Thin Surface
The most prominent feature of thin-walled aluminum alloy components is their extremely thin surface. If CNC machine operators use numerical control machine tools for operations, the inherent elasticity of thin plates, coupled with the interaction of forces during cutting, can cause vibration issues on the cutting surface. This, in turn, makes it challenging to effectively control the thickness and dimensions of the cutting surface, thereby increasing the surface roughness of thin-walled aluminum alloy components.

Processing methods?of aluminum alloy
Hot Working
Hot working refers to the plastic forming process completed above the recrystallization temperature when feeding aluminum alloy ingots. During hot working, the ingot’s plasticity is high, and the deformation resistance is low, allowing the production of larger products with smaller equipment capabilities.
Cold Working
Cold working refers to the plastic forming process completed below the temperature that induces recovery and recrystallization. The essence of cold working is a combination of cold working and intermediate annealing processes. Cold working can produce final products with smooth surfaces, precise dimensions, good structural properties, and the ability to meet various performance requirements.
Warm Working
Warm working is a plastic forming process that falls between cold and hot working. The primary purpose of warm working is to reduce the deformation resistance of the metal and enhance its plasticity.
Selection method of cutting aluminum alloy cutting tool
Due to the extremely sharp cutting edges and grooves of solid carbide tools, they exert low cutting forces in precision machining of aluminum alloys. They offer advantages such as large chip space and smooth chip evacuation. Consequently, solid carbide tools have gradually replaced traditional high-speed steel tools.
Aluminum alloy is easily machinable, allowing for higher cutting speeds suitable for high-speed machining. However, due to the low melting point of aluminum alloy, its plasticity increases with temperature. Under high-temperature and high-pressure conditions, significant frictional forces occur at the cutting interface, making it prone to tool adhesion. This is especially true for annealed aluminum alloys, which make it challenging to achieve a small surface roughness.
To obtain a smooth workpiece surface, a combination of rough and finish cutting is often employed. This is because various qualified workpiece blanks tend to have some oxide layers, causing considerable wear on the cutting tools. If the final cutting operation uses polished sharp tools for fine cutting, the above requirements can be met.
When selecting suitable tool materials for aluminum-silicon alloys, the silicon content guides the choice. For silicon content below 12%, tungsten steel tools in the ISO K10-K20 range can be used. If the silicon content exceeds 12%, diamond tools are preferred. Alumina ceramic tools are not suitable for aluminum alloy processing. During cutting, the oxidized aluminum chips can chemically bond with the ceramic tool, causing adhesion and chip lumps, leading to increased friction resistance and accelerated wear. Once chip lumps form, they replace the cutting edge during machining. In ultra-precision machining, the sharpness of the tool edge loses its significance. Additionally, the bottom of the chip lump is relatively stable, while the top is unstable and prone to breakage. After breaking, part of it is expelled with the chips, while the remaining part stays on the machined surface, making it rough. The protruding part of the chip lump beyond the tool edge also directly contributes to roughening the machined surface, and the friction between the chip lump and the already machined surface further increases surface roughness.
]]>What is carbide?
carbide?is an alloy material made through powder metallurgy process, consisting of hard compounds of refractory metals and a binding metal. carbides exhibit excellent properties such as high hardness, wear resistance, good strength and toughness, heat resistance, and corrosion resistance. Particularly noteworthy are their high hardness and wear resistance, which remain largely unchanged even at temperatures as high as 500°C and maintain significant hardness at 1000°C.
carbides are widely used as cutting tool materials, including turning tools, milling cutters, planers, drill bits, boring tools, etc. They are employed for machining a variety of materials, including cast iron, non-ferrous metals, plastics, synthetic fibers, graphite, glass, stone, and common steel. Additionally, carbides can be utilized for cutting challenging materials like heat-resistant steel, stainless steel, high manganese steel, and tool steel.
?
What is metal ceramic?
Metal ceramic is a composite material composed of ceramic and metal. It is defined by the ASTM (American Society for Testing and Materials) committee as a heterogeneous composite material consisting of metal or alloy and one or more ceramic phases, where the latter typically constitutes 15% to 85% by volume. Importantly, at the preparation temperature, there is minimal solubility between the metal and ceramic phases. In a narrow sense, metal ceramics refer to a category of materials within composite materials where both metal and ceramic phases have interfaces in three-dimensional space.
Composition of metal ceramic
Metal ceramics are created by adding metal powder to the clay used in ceramic production, allowing the ceramic to withstand high temperatures without becoming easily breakable. Metal matrix metal ceramics, also known as dispersion-strengthened materials, are produced by adding oxide fine powders to a metal matrix. Examples include sintered alumina (aluminum-alumina), sintered beryllium (beryllium-beryllium oxide), TD nickel (nickel-thorium oxide), and others. These are composite materials composed of one or more ceramic phases and metal or alloy phases.
In a broader sense, metal ceramics also encompass refractory compound alloys, carbides, and metal-bonded diamond tool materials. The ceramic phase in metal ceramics consists of oxides or refractory compounds with high melting points and high hardness, while the metal phase primarily consists of transition elements (iron, cobalt, nickel, chromium, tungsten, molybdenum, etc.) and their alloys.
Classification of metal ceramic
Metal ceramics are classified into two categories based on the percentage of each component phase: those with ceramics as the matrix and those with metals as the matrix. Metal matrix metal ceramics typically exhibit high temperature strength, low density, ease of processing, corrosion resistance, and good thermal conductivity. Therefore, they are commonly used in the manufacturing of structural components for aircraft and missiles, engine pistons, chemical machinery parts, and more.
Ceramic matrix metal ceramics can be further subdivided into several types:
Oxide-based metal ceramics
Used for missile nozzle liners, crucibles for melting metals, and metal cutting tools.
Carbide-based metal ceramics
Utilized in the production of cutting tools, high-temperature bearings, sealing rings, wire drawing dies, and turbine blades.
Nitride-based metal ceramics
Less commonly applied.
Boride-based metal ceramics
Less commonly applied.
Silicide-based metal ceramics
Using silicides as the matrix, combined with partial or trace amounts of metal materials. Among them, siliconized molybdenum metal ceramics find wide applications in industry.

The main difference between the two materials
Material distinction
WC (tungsten carbide) carbide?blades are produced using advanced metal powders such as tungsten-cobalt, tungsten-samarium, tungsten-titanium, tungsten carbide, etc., as raw materials, and they undergo high-temperature and high-pressure sintering to achieve their final form. On the other hand, ceramic blades use raw materials like aluminum oxide, zirconium oxide, etc., and are formed through high-temperature sintering treatment to create a new type of material.
Cutting performance
Hardness
Ceramic blades typically exhibit higher hardness than WC (tungsten carbide) carbide?blades, reaching levels of 1800-2200HV, while the hardness of WC carbide?blades generally falls between 1600-2000HV.
Cutting Performance
In comparison to WC carbide?blades, ceramic blades offer higher precision and smoother cutting surfaces. Achieving high-precision machining results often requires only a single cut with ceramic blades. WC carbide?blades perform better in cutting softer materials, and they often have faster cutting speeds.
Cutting Lifespan
Ceramic blades have better wear resistance, resulting in a longer lifespan. WC carbide?blades are typically more suitable for mass production processing of workpieces.

Physical performance
Hardness
Ceramic blades typically exhibit higher hardness than WC (tungsten carbide) carbide?blades, reaching levels of 1800-2200HV, while the hardness of WC carbide?blades generally falls between 1600-2000HV.
Cutting Performance
In comparison to WC carbide?blades, ceramic blades offer higher precision and smoother cutting surfaces. Achieving high-precision machining results often requires only a single cut with ceramic blades. WC carbide?blades perform better in cutting softer materials, and they often have faster cutting speeds.
Cutting Lifespan
Ceramic blades have better wear resistance, resulting in a longer lifespan. WC carbide?blades are typically more suitable for mass production processing of workpieces.
The differences between the two materials
Machining Performance
Ceramic blades are relatively brittle and prone to fracture under external impact. In contrast, the manufacturing process for WC (tungsten carbide) carbide?blades is simple, and they are easy to use and maintain.
Price and Applicability
Ceramic blades are relatively more expensive but are suitable for high-precision cutting processes, such as in the fields of microelectronics and semiconductors. On the other hand, WC carbide?blades are more cost-effective and are suitable for large-scale machining applications.
