1. CVD Diamond Introduction
Chemical Vapor Deposition (CVD) diamond refers to the use of CVD method, under low pressure conditions, with carbon-containing gases such as H2 and CH4 as the reaction gas, chemical reactions under plasma-assisted and certain temperature conditions, resulting in solid particle deposition Diamond obtained on the heated substrate surface. Similar to natural diamond, CVD diamond is a crystal of a single carbon atom and belongs to a cubic system. Each C atom in the crystal forms a covalent bond with sp 4 hybrid orbital and another 4 C atoms, and has strong binding force and stability. Nature and directionality; the bond length and bond angle between C atoms and C atoms are equal, and they are arranged in an ideal spatial network structure, making CVD diamonds exhibit comparable mechanical, thermal, optical, and electrical properties of natural diamonds. Comprehensive performance
As we all know, natural diamond reserves in the natural world, mining costs are high, the price is expensive, it is difficult to widely promote the application in the industrial field. Therefore, the synthesis of diamond by artificial methods such as high temperature and high pressure (HTHP) and CVD has gradually become the main way for people to obtain such excellent materials with excellent properties. Diamond products synthesized by HTHP method are generally in the state of discrete single-crystal particles. Although HTHP method has been able to synthesize large single crystals with diameters larger than 10 mm with the development of science and technology, the current products are still mostly single crystals with a diameter of 5 mm or less. And mainly diamond powder. In contrast, the size of the diamond single crystal synthesized by the CVD method is determined by the size of the seed crystal, and a larger-sized diamond single crystal can also be obtained by using multiple growth and “mosaic” growth methods. In addition, the CVD method can also be used to prepare large-area diamond self-supporting films by heteroepitaxial deposition or to coat diamonds on the surface of various complex shapes to form a wear-resistant or protective coating, which greatly expands the application of diamond. It can be seen that CVD diamond has a very wide range of application prospects in many fields such as machining, defense and nuclear industry. Among them, the application in the machining industry mainly includes grinding wheel dressers, trimming pens, various cutting tools, etc. When used in these aspects, only the hardness, wear resistance, and chemical stability of the diamond are involved, and transparency is not required. The properties such as dielectric loss and product preparation are relatively easy, so the application on the tool is the main field of large-scale industrial application of CVD diamond.
2. CVD Diamond Coated Carbide Tools
Diamond cutters currently on the market mainly include single crystal diamond tools, polycrystalline diamond (PCD) tools, diamond thick film welding tools, and diamond coated tools. The latter two are applications of CVD diamond as a tool. Among them, the diamond thick film welding tool is generally prepared by cutting a CVD self-supporting diamond thick film with a thickness of 0.3 mm or more and then welding it onto a substrate. Because diamond thick films can be cut into any two-dimensional shape, they are less expensive and more flexible than single-crystal tools. In addition, Co-bonds are not included in diamond thick films compared to PCD tools. High machining accuracy and high wear ratio.
For diamond-coated tools, the CVD method is used to apply a diamond coating less than 30 μm thick on the surface of the tool body. Compared with the other three tools, the CVD method can apply diamond to tools with complex shapes including various drills, milling cutters, etc.; and since the diamond coating is thin and the deposition time is short, the coated tool does not need to follow up. Processing, so the cost is low.
Therefore, the current tool market analysis generally believes that CVD diamond-coated tools will be one of the most important development directions of the tool industry. Of the many tool materials, WC-Co cemented carbide is the most widely used. It not only has high hardness, excellent thermal stability, but also has high strength and good toughness. It is the ideal diamond coating. Layer tool base material. The CVD diamond-coated CVD diamond-coated carbide cutting tools prepared from CVD diamond on the surface of WC-Co cemented carbide can perfectly combine diamond’s excellent wear resistance, heat dissipation, and good toughness of cemented carbide. Effectively solve the contradiction between the hardness and toughness of existing tool materials, and greatly improve the cutting performance and service life of carbide tools. In the non-ferrous metal and its alloys, various particles or fiber reinforced composite materials, high-performance ceramics and other materials processing The field has a broad application prospects.
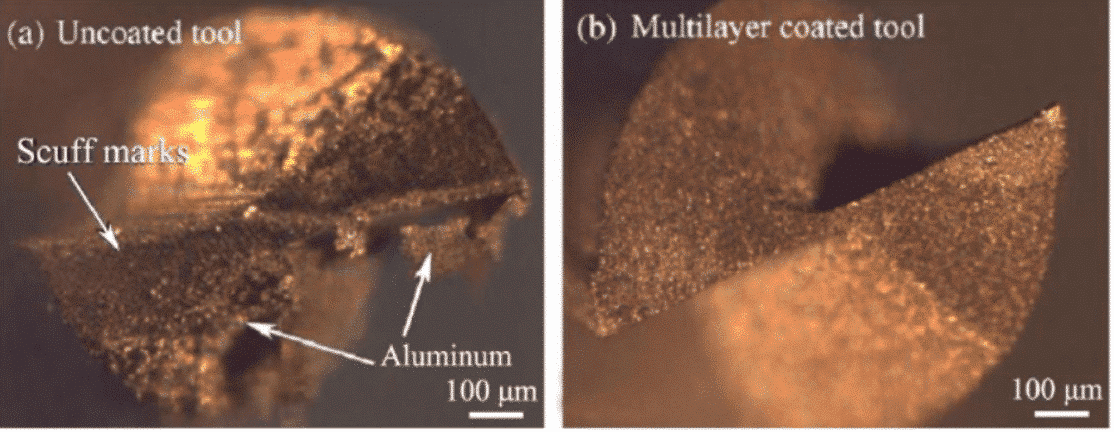
Fig. 1 Cutting edges of (a) the uncoated tool and (b) diamond coated tool after cutting tests
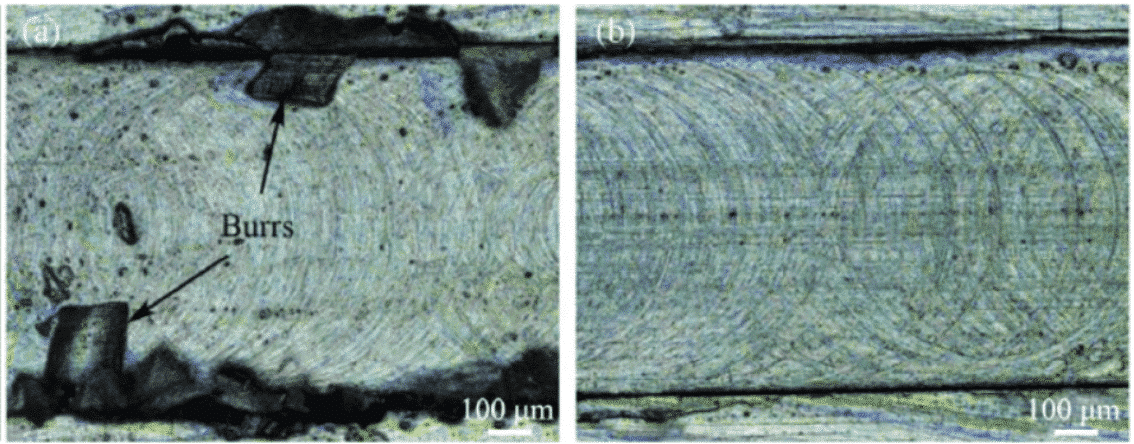
Fig. 2 Representative end milled channels in Al alloy after being cut by (a) uncoated tool and (b) diamond coated tool
In summary, diamond-coated carbide tools exhibit excellent performance in terms of turning, milling, and drilling. For example, the wear of the cutting edge is small, the service life is long, and the machining is not “sticking” and High processing accuracy. Therefore, compared with other tools, diamond-coated carbide tools can better meet the processing requirements of current new materials and ultra-precision cutting.
3. Problems and Solutions of CVD Diamond Coated Carbide Tools
Although a large number of research results have shown that CVD diamond coated carbide tools have excellent performance and long service life, there are also reports of successful production trials by some manufacturers at home and abroad. But so far, this tool has not been applied in large-scale industrial production. The main reason is that currently produced diamond-coated tools still have problems such as low bonding strength between the coating and the substrate, large surface roughness of the diamond coating, and poor quality stability. Among them, the low bond strength of the coating is a key technical obstacle that limits the large-scale application of this tool.
The primary reason for the low bonding strength of diamond coatings is the presence of Co-bonded phases in cemented carbide substrates. At CVD diamond deposition temperatures (600 ~ 1200 °C), Co has a high saturation vapor pressure, will rapidly diffuse to the substrate surface, inhibit diamond nucleation and growth, and catalyze the formation of graphite and amorphous carbon, leading to diamond coating and The bond strength between cemented carbide substrates is reduced. In addition, the difference in physical properties such as lattice constant, hardness, and coefficient of thermal expansion (CTE) between diamond and cemented carbide materials is also a major cause of the low bonding strength of the coating.
Diamond is a face-centered cubic crystal with a lattice constant a0 = 0.35667 nm, a hardness of 60 ~ 100 GPa, and a CTE of 0.8 ~ 4.5 × 10-6 /°C. The cemented carbide consists mainly of WC particles and a Co binder. WC For the close-packed hexagonal crystal structure, the lattice constant a = 0.30008 nm, c = 0.47357 nm, the hardness of the cemented carbide is approximately 17 GPa, and the CTE is approximately 4.6×10-6 /°C. These differences will result in diamond coating and The thermal stress at the interface of the cemented carbide substrate is not conducive to the adhesion of the diamond coating on the cemented carbide substrate.
A large number of studies have shown that pretreatment of the surface of the cemented carbide substrate to reduce the adverse effect of the Co binder on the deposition of the diamond coating is the most effective method for improving the bonding strength of the diamond coating/cemented carbide substrate. The current major pretreatment methods include:
(1) Surface Removal Co Treatment
This method usually adopts physical or chemical means to remove the Co of the surface layer of WC-Co so as to suppress or eliminate its negative influence and improve the bonding strength between the diamond coating and the substrate. Among them, the most widely used in the industry is the “acid-base two-step method”, which uses the Murakami solution (1:1:10 KOH+K3[Fe(CN)6]+H2O) to corrode the WC particles and roughen the hard alloy. The surface was then etched using Caro acid solution (H2SO4 + H2O2) to remove the surface Co. This method can inhibit the negative catalytic effect of Co to a certain extent and improve the bonding strength of the diamond coating. However, after processing, it will form a loose zone near the substrate near the surface layer, reduce the fracture strength of the coated tool, and the Co The higher the content of the binder, the more severe the impact on the tool performance.
(2) Apply a transition layer method
The method is to prepare one or more layers of transition layers between the diamond coating and the cemented carbide substrate for blocking out diffusion of Co and suppressing its negative catalytic effect on diamond deposition. Through reasonable material selection and design, the prepared transition layer can also reduce the abrupt change of the physical properties of the interface, and reduce the thermal stress caused by the differences in physical properties such as CTE between the coating and the substrate. The application of the transition layer method generally does not cause damage to the surface layer of the substrate, nor does it affect the mechanical properties such as the fracture strength of the coating tool, and it can prepare CVD diamond coatings on high Co content cemented carbides, and therefore is currently researching and improving WC- The preferred method of bonding the diamond coating on the Co substrate surface.
4. Selection of transition layers and preparation methods
According to the previous analysis, the application of the transition layer method can effectively suppress the negative catalytic effect of Co, and will not damage the matrix. However, to effectively achieve the function of increasing the bonding strength of the diamond coating, the material selection and preparation method of the transition layer is very important. The selection of transition layer materials generally requires following several principles:
(1) It has good thermal stability.
The deposition temperature of the diamond coating is generally 600 ~ 1200 °C, the transition layer material can withstand higher temperatures, does not occur softening and melting;
(2) Hardness and CTE properties are best placed between diamond and cemented carbide to reduce the thermal stress caused by mismatching performance;
(3) Prevents Co from migrating to the surface during diamond deposition or reacts with Co to form stable compounds;
(4) It has good compatibility with diamond materials. Diamond can nucleate and grow on the surface of the transition layer. In the nucleation stage, diamond can rapidly nucleate and have a high nucleation rate.
(5) The chemical properties are stable and have a certain mechanical strength, so as to avoid the formation of a soft intermediate layer and adversely affect the performance of the coating system.
At present, people study and use more transition layers mainly include metals, metal carbon/nitrides, and composite transition layers composed of them. Among them, Cr, Nb, Ta, Ti, Al and Cu are generally used as the transition layer materials for the metal transition layer, and the PVD, electroplating, and electroless plating are commonly used as the preparation methods, and the PVD method is most widely used. The results show that the transition layer formed by the carbon-philic metal is more effective in improving the bonding strength of the diamond coating than the weak carbon metal. In the initial stage of diamond deposition, a layer of carbide is first formed on the surface of the metal layer, and this layer of carbide facilitates the nucleation and growth of the diamond. However, the metal transition layer has a large CTE and a high requirement for the thickness. If it is too thick, it will lead to an increase in thermal stress, decrease the bonding strength, and be too thin to completely block the outward diffusion of Co. In addition, the metal transition layer is relatively soft, which is equivalent to adding a soft layer in the middle of the hard phase, which is not conducive to the matching degree of the coating system performance.
The hardness of the carbon/nitride transition layer is higher than that of the pure metal, and there is no problem of reducing the use performance of the coated tool. WC, TiC, TaC, TaN, CrN, TiN, and SiC are currently the most studied and used transition layer compounds. Such transition layers are generally prepared by reactive magnetron sputtering and other methods. Studies have shown that the carbon/nitride transition layer can effectively block the out-diffusion of Co, and thus can improve the bonding strength of the diamond coating to some extent. The degree of improvement of bonding strength of such transition layers generally depends on the matching of the CTE of the transition layer with the matrix and the diamond, the structure of the transition layer, and the wettability of the transition layer material and the diamond.
Common metal carbides have a lower CTE than metal nitrides, and when carbide transition layers are used, diamonds can be nucleated directly on the transition layer, which shortens the nucleation time compared to metal transition layers and nitride transition layers. From this we can see that carbides are one of the more ideal transition layer materials. Among these metal carbide materials, HfC, NbC, Ta C, and the like have a relatively low CTE. In addition, the non-metallic carbide SiC has the lowest CTE in all carbides (β-SiCCTE = 3.8×10-6/°C), which is between the cemented carbide and diamond. Therefore, there are many studies on the SiC transition layer. For example, Cabral G and Hei Hongjun used CVD method to prepare SiC transition layer on the surface of cemented carbide for deposition of diamond coating. The results show that SiC transition layer can effectively enhance the bonding between diamond coating and cemented carbide substrate.
Intensity, but the CVD method directly prepared SiC coating on the surface of the cemented carbide, the content of Co binder phase in the cemented carbide substrate is not easy to be too high (generally <6%), and the deposition temperature needs to be controlled in a low range (generally 800 °C or so). This is mainly due to the fact that the catalytic action of the Co-binder phase is significant at high temperatures, resulting in the formation of SiC whiskers, and there is a large amount of voids between the whiskers and cannot be used as a transition layer. However, at low deposition temperatures, loose amorphous SiC coatings are prone to occur. Therefore, a deposition temperature range that is dense, continuous, and satisfies the use as a buffer layer of the SiC coating layer is made smaller. Therefore, when some researchers use SiC as a transition layer, in order to obtain high bonding strength, it is necessary to first use etching to remove Co in the hard alloy layer. Therefore, the catalytic action of Co has become one of the key factors limiting the use of SiC as a transition layer.
The composite transition layer is generally a multi-layer coating composed of a combination of two or more kinds of metal or metal carbon/nitride materials. At present, there are many composite transition layers including W/Al, W/WC, CrN/Cr, and ZrN/. Mo, TaN-Mo, and 9x (TaN/ZrN)/TaN/Mo, etc., are also mostly PVD or CVD methods. Such transition layers generally include a Co diffusion barrier layer and diamond-like nucleation promoting layer, that is, the functional requirements of the transition layer are fully satisfied by using a reasonable multilayer material. Compared with the single metal transition layer and the carbon/nitride transition layer, the composite transition layer is more conducive to improving the bonding strength between the diamond coating and the cemented carbide substrate. However, in order to obtain a composite transition layer with excellent performance, it is generally necessary to perform reasonable material selection and design. Otherwise, the expected effect may not be achieved because of large differences in the physical properties of the materials or the increased number of interfaces.
From the perspective of the preparation method of the transition layer, currently, researchers mostly use physical vapor deposition (PVD), electroplating, electroless plating, and CVD to prepare the transition layer. The obtained transition layer and the matrix are usually physically bound or only existed. A nanometer-thick diffusion layer, which adds one or more new interfaces between the diamond coating/cement substrate. A sudden change in physical properties such as CTE and hardness between the transition layer material and WC-Co will also cause interfacial stress problems, and this interfacial stress will increase with the increase of the thickness of the transition layer and the number of transition layers, affecting to some extent. Increased bonding strength. Furthermore, apart from SiC, there are still large differences in properties such as CTE and hardness between other transition layer materials and diamonds, which is not conducive to the improvement of bonding strength. Therefore, to explore a new preparation method of the transition layer, to obtain a transition layer with a gradient of composition and composition, and to avoid the interface stress caused by the new interface, it is particularly important to enhance the bonding strength of the diamond coating.









